A blog about how halophytic plants might save the future of agriculture
in the San Joaquin Valley
David Gallagher
Why does agriculture
need to be saved in the San Joaquin Valley?
The San Joaquin Valley contains seven of the top ten
counties in California in terms of total value of crops produced. In 2007, an
estimated $25 billion of crop commodities were produced in the San Joaquin
Valley. Some important crops are grapes, oranges, cotton, almonds, walnuts, and
cherries. It has been called “The food basket of the world”. However, the
productivity and sustainability of the San Joaquin Valley is threatened by the
build up of salt in the groundwater that has occurred over the last 60 years. Hydro-salinity
models have suggested that irrigation in the San Joaquin Valley is not
sustainable and salinity will continue to rise because of the closed
groundwater systems (systems that do not have sufficient leeching of the
groundwater to remove excess salt). Salinity has increased primarily because of
evaporation and transpiration (the movement of water within a plant from the
roots to the leaves and into the air as water vapor) of pure water from the
soil solution, thereby concentrating salt in the groundwater. Irrigation with fresh
water does not solve the problem because the increase in the water table
results in the capillary rise of the already high-salinity groundwater into the
rooting zones, thereby increasing salt concentrations in the shallow
groundwater zone. One of the big problems for San Joaquin Valley agriculture is
that high salt concentrations are toxic to most agricultural crops.
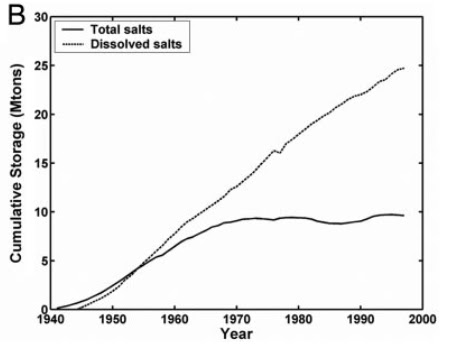 |
| Change in total salt storage and dissolved salts (in megatons) since 1940 in a 1,400 square km area in western Fresno County on the western side of the San Joaquin Valley |
 |
| Salt deposits on land formerly used for agriculture in the San Joaquin Valley, CA http://ucce.ucdavis.edu/files/repository/calag/img6301p42.jpg |
What is too salty?
The presence of ions in the groundwater is expressed in
terms of the electrical conductivity of a soil-saturated extract (ECe)
and is expressed in decisiemens (siemens is a unit of electrical conductance)
per meter (dS/m). You might recall that sodium chloride (salt) dissociates in
water into Na+ and Cl- ions.
The more ions dissolved in water, the better the water conducts electricity. Of
course, other ions are present, but in the San Joaquin Valley salt ions are
generally responsible for the electrical conductivity of the soil. Values for
ECe range form less than 1 dS/m to as high as 16 dS/m for the San
Joaquin Valley depending on the depth of the measured groundwater. The deepest
parts (20-40 meters) of the groundwater system have the smallest ECe values and the shallower parts (6
meters or less) of the groundwater system have the largest ECe values.
In general, when the ECe values exceed 2.0 dS/m, many plants start
to experience stress. Some typical threshold values (the highest value of
electrical conductivity the plant can grow in) for ECe for various
crops are almonds at 1.5 dS/m, grapes at 1.5 dS/m, and walnuts at 1.0 dS/m.
Why is salt toxic to
plants?
The nutrient status of a plant is disrupted because salt
ions can easily move through the vascular tissue of the plant by way of the
transpirational stream (the movement of water throughout the plant). Under
normal conditions the cytosol of higher vascular plants (all agricultural
plants) contain approximately 10mM Na+. Under saline stress,
cytosolic salt ions increase to more than 100mM and become cytotoxic. The high
concentrations of salt ions result in protein denaturation and membrane
destabilization and ultimately the disruption of biochemical pathways essential
to photosynthesis and sugar production. High salt concentrations also result in
osmotic stress.
How do plants respond
to salty environments?
In addition to the toxic effects of salt, salt also
decreases the water potential of the soil around roots, resulting in osmotic
stress. Plants can only continue to absorb water if the water potential at the
leaf surface is more negative than the water potential in the soil. This is
analogous to sucking water up through a straw from a glass - your intake of air
is creating a negative pressure at the top of the straw relative to the
pressure at the bottom of the straw and the resulting pressure differential
results in the movement of water up the straw. Plant physiologists call these
pressures water potentials. Plants use the difference in water potential
between the roots and the leaves to maintain turgor pressure (think about it in
terms of water pushing against the inside of the cell wall trying to get out)
and it is what maintains a plant’s rigidity, and ultimately allows for growth. The
reason that salt lowers the water potential is that as the salt concentration
in the soil increases, the amount of water in the soil decreases. When the
amount of water in the roots becomes greater than in the soil (roots are
relatively permeable to water), the water wants to move into the soil. This
movement of water is called osmosis.
Most plants can overcome a moderate increase in salt
concentration and the resulting change in water potential because they can make
osmotic adjustments (osmoregulate) in response to their environment.
Osmoregulation is the ability of plant cells (or most cells for that matter) to
adjust the osmotic concentrations of osmolytes (molecules that affect the
movement of water) within their cells to maintain the needed amount of water in
the cytosol and the external environment. The movement of Na+ and Cl-
ions during osmoregulation takes place mainly in the vacuoles
(an organelle of a plant cell that forms a closed compartment within the cell).
When ions are compartmentalized in the vacuole, other solutes (osmolytes) must
accumulate in the cytosol to maintain the proper water potential or water will
diffuse out of the cell (remember we want the water to come into the cell to
maintain turgor pressure). Many plants use organic compounds as osmolytes, such
as proline (an amino acid), sorbitol, glycine betaine, and
3-dimethylsulfoniopropionate (DMSP). The reason plants use these compounds is
that the cell can maintain large concentrations without experiencing
detrimental effects on metabolism. Some of these compounds, such as proline actually
protect the cell from toxic byproducts during periods of stress, such as
periods of water shortage or osmotic stress.
What is a halophytic
plant?
Plants that are adapted to grow in saline environments are
called halophytes. Halophytes have a greater capacity for vacuolar
sequestration of sodium and chloride ions in leaf cells. They also posses the
ability to reduce cellular uptake of Na+ across the cellular
membrane. These two features allow halophytes to tolerate a higher Na+
in the roots and in the transpirational stream. A large part of salt tolerance
in halophytes stem (pun intended!) from the synthesis of additional vacuolar
transporters. Plant cells need a way to move ions from the cytosol into the
vacuole. This movement is accomplished by trans- membrane proteins (they span
the width of the plasma membrane and form a sort of bridge from the inside to
the outside of the cell) that are specialized in moving specific ions in
specific directions. This specificity can thought as bridge that only allows
one type of car to cross. These transporter proteins are critical for sequestering
Na+ and Cl- ions. You can see from the diagram below that
the cell uses a combination of primary and secondary active transporters. The
primary transporters uses ATP (the energy currency of a cell) to establish a
proton (H+ ions) gradient and then the secondary
transporters use the proton gradient (the protons are now moving down their
concentration gradient) to move Na+ ions against their concentration
gradient by coupling the two movements together.
 |
| Primary and active transport for a typical plant cell. |
Other halophytes, such as Atriplex spp. (saltbush) have
evolved specialized hairs on the surfaces of their leaves that excrete salt. Saltbush
is found worldwide in saline environments, from estuaries to inland salt sinks
and can even tolerate irrigation with seawater. The specialized hairs are
called vesiculated trichomes. Each trichome has a stalk and a balloon-like tip
called the bladder cell. The leaves sequester the salt ions in the bladder
cells and when ruptured, release salt back into the environment. The plant
actively transports Na+ ions
and Cl- ions into the
vacuoles of the bladder cells using the mechanism previously described. The
leaves of Atriplex have a silvery
reflectance due to the presence of the trichomes.
 |
| Atriplex leucophylla in Morro Bay, CA showing the silvery reflectance of the trichomes http://calphotos.berkeley.edu/cgi-bin/img_query?rel-taxon=begins+with&where-taxon=Atriplex+leucophylla |
GMO’s to the rescue?
Can the adaptations that halophytes utilize to grow and
thrive in saline environments be used to improve the salt tolerance of
agricultural crops? Genetically modified organisms or in our case,
plants are widely used in agriculture today.
Since the first genetic modified tomato plant became available for
commercial sales in 1994, genetic engineering has proven a revolutionary
technique to generate desirable traits in plants. GMO’s differ from
conventional bred crop varieties in that a specific gene or genes can be
transferred from any organism (not only a plant) to the host plant without the
need for crossbreeding and the genes introduced are often genes that could
never be crossed successfully. These genetically modified plants are referred
to as transgenic plants. Transgenic plants include “golden rice”, a rice with
large amounts of B-carotene that was created to reduce Vitamin A deficiency in
children in many parts of the world. Also, there is a transgenic corn that is
resistant to the herbicide Roundup and is widely planted in the United States.
The benefit is that the corn fields can be treated with Roundup, killing only weeds
and thereby increasing crop yields.
There are
encouraging results in terms of improved salinity tolerance (by inserting genes
that are known to impart improved resistance to salinity) in various crop plants
(under controlled laboratory conditions) through the use of genetic
engineering. However, there is further need of improvement for successful release
of salt tolerant plants that will be successful in the field under real-world
growing conditions. As you can see from the diagram below, there are many genes
that have been identified as imparting salinity tolerance to different plants. In the real world, it probably will not involve just one
gene that can be inserted into a crop of choice, but ultimately many genes.
 |
| Known genes that impart salinity tolerance to different plants |
Recently,
researchers in Australia were able to cross Triticum
monococcum (einkorn wheat), one of the earliest cultivated species of salt
tolerant wheat with Triticum durum (durum
wheat), a commercially important and widely cultivated wheat that is used to
make pasta. Traditional cross breeding methods were used and the process took
over 15 years to complete. The new line outperformed the commercial variety by approximately 25% in
salty soils in real world conditions of commercial agriculture. Even though
traditional methods of crossbreeding were used, it was the knowledge from
transgenic plants that allowed an understanding at the molecular level of what
was going on. It was determined that the new variety of wheat had a gene for a particular
trans-membrane sodium transporter protein. This protein was found in root cells
that surround the xylem (vascular system) and it actively pumps sodium from
water in the xylem before the water travels to other parts of the plant.
The future
of salt tolerant crops might just involve a combination of the traditional
techniques of crossbreeding and the modern techniques of genetic modification.
It is possible that the next time you drive by a grove of almond trees in the
San Joaquin Valley the leaves might be shimmering with a silvery reflectance.
References
Apse, M.P., and E. Blumwald. 2007. Na+ transport in plants. FEBS Letters
581:2247-2254.
California Agricultural Resource Directory 2008-2009. Agriculture
Statistical Review.
Hasegawa, P.M., R.A. Bressan, J.K. Zhu, and H.J. Bohnert.
2000. Plant cellular and molecular responses to high salinity. Annual Review of
Plant Physiology and Plant Molecular Biology 51:463-499.
Hoffman, G.J. 2010. Salt tolerance of crops in the southern
Sacramento-San Joaquin Delta. California Environmental Protection Agency State
Water Resources Board Division of Water Rights, Final Report.
Munns R., R.A. James, B. Xu, A. Athman, S.J. Conn, C.
Jordans, C.S. Byrt, R.A. Hare, S.D. Tyerman, M. Tester, D. Platt, and M.
Gilliham. 2012. Wheat grain yield on saline soils is improved by an ancestral
Na+ transporter gene. Nature
Biotechnology 30:360-364.
Schoups, G., J.W. Hopmans, C.A. Young, J.A. Vrugt, W.W.
Wallender, K.K. Tanji, and S. Panday. 2005. Sustainability of irrigated
agriculture in the San Joaquin Valley, California. Proceedings of the National
Academy of Sciences 102:352-356.
Smaoui, A., Z. Barhoumi, M. Rabhi, and C. Abdelly. 2011.
Localization of potential ion transport pathways in vesicular trichome cells of
Atriplex halimus L. Protoplasma
248:363-372.
Taiz, L., and E. Zeiger. 2010. Plant Physiology 5th
Edition. Sinauer Associates, Sunderland, MA.
Turan, S., K. Cornish, and S. Kumar. 2012. Salinity
tolerance in plants: Breeding and genetic engineering. Australian Journal of
Crop Science 6:1337-1348.


No comments:
Post a Comment